Medicines and Healthcare products Regulatory Agency
783 articles
Showing 451-480

Skin creams dried on fabric can lead to fire deaths
Wednesday, 29 July 2020
A new campaign to raise awareness of the fire risk and the precautions that need to be taken by users of skin creams has been launched today...

New synthetic datasets to assist COVID 19 and cardiovascular research
Wednesday, 29 July 2020
Ground-breaking innovation to support medical technologies ...

Medical Device “Certificates of Compliance” / “Attestation of Conformity” have no legal standing under UK Medical Device Regulations 2002
Thursday, 23 July 2020
The MHRA is aware of certificates being issued by certification bodies headed “certificates of compliance” or “Attestation of Compliance”. ...

MHRA response statement to IMMDS Review report publication
Wednesday, 08 July 2020
Today’s publication of the Independent Medicines and Medical Devices Safety Review is of profound importance for the MHRA, since the safety of the pub...
Don’t rely on temperature screening products for detection of coronavirus (COVID 19), says MHRA
Friday, 03 July 2020
Warning that thermal cameras and other such “temperature screening” products, some of which make direct claims to screen for COVID-19, are not a relia...

Chloroquine and Hydroxychloroquine not licensed for coronavirus (COVID 19) treatment
Friday, 26 June 2020
Recent media reports have suggested that chloroquine can protect patients from coronavirus or treat COVID-19, the illness caused by a coronavirus. ...

Green light for COVID 19 trial recruitment
Friday, 26 June 2020
The MHRA has approved the recruitment of further participants for a clinical trial by the University of Oxford...

MHRA suspends recruitment to COVID 19 hydroxychloroquine trials
Tuesday, 16 June 2020
The Medicines and Healthcare products Regulatory Agency (MHRA) has instructed UK clinical trialists using hydroxychloroquine to treat or prevent coron...

David Noakes: Judge orders seizure of £1.4 million
Friday, 05 June 2020
A convicted fugitive faces the confiscation of £1.4 million in prime assets by the Crown, following a hearing at Southwark Crown Court today....

Action taken to halt sales of fingerprick coronavirus (COVID 19) antibody testing kits
Friday, 29 May 2020
Anyone supplying these types of tests should temporarily stop this service....

MHRA issues a scientific opinion for the first medicine to treat COVID 19 in the UK
Tuesday, 26 May 2020
MHRA has today given the first positive scientific opinion under the Early Access to Medicines Scheme (EAMS) for use of Gilead’s remdesivir....

Patients informed to exchange Emerade 500 micrograms adrenaline pens for a different brand
Monday, 18 May 2020
Allergy patients who carry Emerade 500 microgram adrenaline auto-injector pens should contact their prescriber and seek replacement pens of a differen...

Applications invited for the role of Chair of the MHRA
Monday, 11 May 2020
Ministers are seeking to appoint a Chair of the Medicines and Healthcare products Regulatory Agency (MHRA). ...

Senior Enforcement Advisor at UK Medicines Regulator warns against purchasing fake or unlicensed coronavirus (COVID 19) medicines
Thursday, 07 May 2020
Lynda Scammell, Senior Enforcement Advisor at the Medicines and Healthcare products Regulatory Agency (MHRA) today warned the public about medicines a...
New reagent available to support global diagnostic testing of coronavirus (COVID 19)
Wednesday, 06 May 2020
A new biological reagent is now available to labs around the UK and the world that will help them to set up and develop accurate diagnostic tests for ...

Coronavirus: new website for reporting medicines side effects and equipment incidents
Monday, 04 May 2020
A new online reporting site, dedicated to reporting?any?suspected side effects?from medicines, future vaccines and incidents involving?medical equipme...

MHRA approves COVID 19 vaccine trial in 7 working days
Thursday, 23 April 2020
We are prioritising and giving tailored scientific advice for potential treatments for coronavirus (COVID-19)....

Patients informed to exchange Emerade 300 micrograms adrenaline pens for replacement pens of a different brand
Tuesday, 07 April 2020
Allergy patients who carry Emerade 300 microgram adrenaline auto-injector pens should contact their prescriber and seek replacement pens of a differen...

Birmingham pair given suspended prison sentences for selling medicines online illegally
Monday, 06 April 2020
A man and a woman who sold breast cancer and erectile dysfunction medicines illegally online appeared at Birmingham Crown Court....

UK medicines and medical devices regulator investigating 14 cases of fake or unlicensed COVID 19 medical products
Friday, 03 April 2020
An increasing number of bogus medical products being sold through unauthorised websites claiming to treat or prevent COVID-19 are being investigated b...

MHRA approves new life saving breathing aid to help keep coronavirus (COVID 19) patients out of intensive care
Tuesday, 31 March 2020
Adapted breathing aid developed by UCL, UCLH and Mercedes Formula One provides vital technology to NHS....
Status of Phase 1 clinical trials in response to coronavirus (COVID 19)
Tuesday, 31 March 2020
We have written to all Phase I accredited units conducting early phase clinical trials to seek confirmation that all trials have undergone a risk asse...

MHRA services during the Coronavirus (COVID 19) response
Friday, 27 March 2020
How to get in touch with the MHRA during this period....

Coronavirus: global crackdown sees a rise in unlicenced medical products related to COVID 19
Thursday, 19 March 2020
The Medicines and Healthcare Products Regulatory Agency (MHRA) took part in the annual global coordinated operation to tackle the illegal online sale ...

Regulator recalls medicine used to treat myomas
Wednesday, 18 March 2020
Patients are urged to stop taking 5mg ulipristal acetate as soon as possible and contact a healthcare professional for advice on alternative treatment...
Patients asked to return Emerade 150 micrograms adrenaline pens
Wednesday, 04 March 2020
Patients, or carers of patients, who carry Emerade 150 microgram auto-injector pens should return all in-date Emerade 150 micrograms auto-injectors to...

New figures show 3.5 million unlicensed erection pills seized in 2019
Tuesday, 03 March 2020
Around 3.5 million unlicensed erection pills worth more than £10 million were seized in the UK in 2019 according to new figures released today by the ...
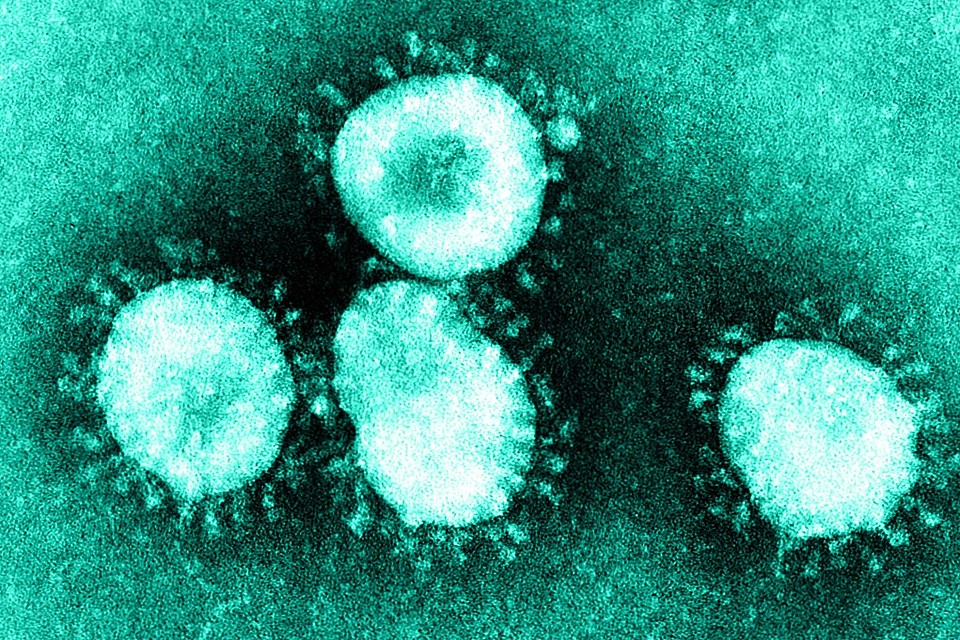
MHRA update on novel coronavirus (COVID 19)
Monday, 24 February 2020
The MHRA is working closely with the DHSC and others in the global response to the new Coronavirus (COVID-19)...

Birmingham pharmacist jailed for trafficking Class C drugs
Thursday, 20 February 2020
A pharmacist was today sentenced to 28 months in prison at the Birmingham Crown Court following an investigation by the MHRA....

Taking multiple medicines? Support the Yellow Card scheme by reporting suspected side effects
Monday, 17 February 2020
The Medicines and Healthcare products Regulatory Agency (MHRA) launches a week long social media campaign to raise awareness about the importance of r...
