Medicines and Healthcare products Regulatory Agency
783 articles
Showing 631-660

Diabetics warned of health risks of suddenly changing life saving devices without medical supervision
Wednesday, 26 October 2016
People with diabetes are being reminded to seek medical advice before changing their insulin therapy devices....

Parents advised not to use unlicensed homeopathic teething products in infants and children
Wednesday, 19 October 2016
Parents of infants and young children should not use unlicensed homeopathic teething tablets or gels which are available to buy online due to the risk...

Promoting biological standardisation – NIBSC and NIFDS sign MoU
Tuesday, 18 October 2016
As part of the Expert Committee on Biological Standardization (ECBS) conference in Switzerland, NIBSC and NIFDS have signed a memorandum of understand...

Teva levothyroxine tablets: re entry to market and introduction of new tablet strengths
Monday, 17 October 2016
Following extensive changes to the formulation and manufacture of levothyroxine tablets by Teva, the Commission on Human Medicines (CHM) is now reassu...

Faulty defibrillator warning
Thursday, 13 October 2016
People and organisations should check if they have the defibrillator models, LIFEPAK CR Plus and LIFEPAK EXPRESS Automatic External Defibrillators (AE...

Improving international collaboration – MHRA and Swissmedic sign MoU
Tuesday, 11 October 2016
As part of the 11th Summit of the Heads of Medicines Regulatory Agencies in Interlaken, the Medicines and Healthcare products Regulatory Agency (MHRA)...

Dodgy dental equipment could come back to bite you
Friday, 07 October 2016
The internet provides easy access to a wide and diverse range of medical products and there has been rapid growth in sites offering cheaper alternativ...

Freshers warned to be smart and avoid Modafinil
Monday, 26 September 2016
MHRA highlight the pitfalls of buying medicines online to students...

Emergency contraceptive affected by other medicines regulator issues new guidance
Thursday, 15 September 2016
Women needing the emergency contraceptive pill containing levonorgestrel should tell their healthcare professional if they are currently taking medici...

Surrey man goes down for selling unlicensed ED drugs and steroids
Wednesday, 14 September 2016
Following an MHRA investigation, a man from Surrey has been jailed for selling potentially dangerous unlicensed medicines....

Dodgy kits give dodgy results
Tuesday, 13 September 2016
Every year the Medicines and Healthcare products Regulatory Agency (MHRA) seize thousands of counterfeit and unlicensed condoms, STI test kits, and HI...
Statement on Statins
Friday, 09 September 2016
Statement from Medicines and Healthcare products Regulatory Agency on the risks and benefits of statins. ...
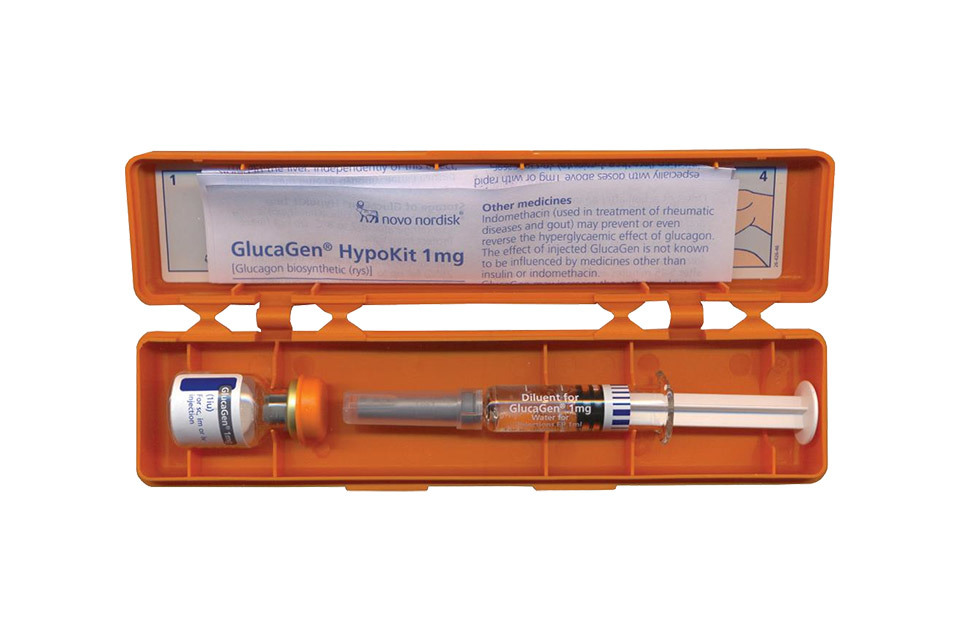
Recall of GlucaGen® HypoKits used by diabetics in an emergency
Tuesday, 06 September 2016
People with diabetes are being asked to check whether they have certain batches of GlucaGen® HypoKits used for the emergency treatment of severe low b...

Manufacturer of Accu Chek Insight insulin pumps issues updated handling instructions following user feedback
Monday, 05 September 2016
People with diabetes using the Accu-Chek Insight insulin pump system should check how they operate and wear the medical device to reduce the risk of a...

Dodgy Datchet ED drugs dealer jailed
Wednesday, 24 August 2016
A Berkshire man has been jailed for the illegal importation and sale of erectile dysfunction medicines...

GCP, GLP and Clinical Laboratories Symposia
Friday, 19 August 2016
19 August 2016...

Dodgy diet pills: Dying to lose weight?
Wednesday, 17 August 2016
MHRA launch FakeMeds campaign with warning on dodgy diet pills...

Avoid risk of high blood glucose: insert pre filled insulin cartridges correctly into insulin pump to manage your diabetes
Tuesday, 16 August 2016
People with diabetes using a particular type of insulin pump and pre-filled insulin cartridges should insert their cartridges correctly to avoid them ...
Medical devices regulator issues alert on faulty glucose test strips
Wednesday, 10 August 2016
People with diabetes who use particular blood glucose test strips should stop using them and seek an alternative as soon as possible says the Medicine...

MHRA review shows sports supplement industry improvement
Tuesday, 02 August 2016
MHRA urges people to be cautious whilst an Olympic review of the sports supplements industry highlights improvements...

MHRA urges people to be cautious when buying sports supplements
Tuesday, 02 August 2016
A review into the sports supplement industry ahead of the Rio 2016 Olympic games shows the sport supplement industry is improving....

Clinical Trials Regulations – have your say
Monday, 01 August 2016
The Medicines and Healthcare products Regulatory Agency invites interested parties to provide feedback on Clinical Trial Regulation guidance documents...
Volunteers wanted to help determine the future of pharmacovigilance
Monday, 11 July 2016
Web-RADR: Recognising Adverse Drug Reactions is running a survey for healthcare providers, patients, and consumers of medicines to help develop the ne...

Regulator issues warning over small number of TRUEyou home use blood glucose test strips
Thursday, 30 June 2016
TRUEyou blood glucose test strips - certain lots of test strips may give incorrect low blood glucose results that could lead to undetected hyperglycae...

Interrogating research to protect public health
Monday, 20 June 2016
Part of the work of the medicines and medical device regulator involves looking at existing research to help reach conclusions about potential and eme...

Man jailed for money laundering and importing and selling unlicensed medicines
Wednesday, 15 June 2016
Tooting man sentenced for importing and selling unlicensed erectile dysfunction medicines, hair loss medicines, and money laundering...

Gang sentenced for importing unlicensed drugs
Friday, 10 June 2016
A gang were sentenced today for their involvement in the illegal importation and sale of erectile dysfunction drugs and tranquilisers....
![[Archived] Draft for comment Human Factors and Usability Engineering – Guidance for Medical Devices Including Drug device Combination Products](https://assets.publishing.service.gov.uk/media/5a61a3dae5274a0a1b10f351/s960_Digital_logo-for-gov-uk.png)
[Archived] Draft for comment Human Factors and Usability Engineering – Guidance for Medical Devices Including Drug device Combination Products
Friday, 10 June 2016
MHRA has produced draft guidance for medical devices including drug-device combination products and we welcome your comments...

MHRA information on TPP and QRISK®2
Thursday, 09 June 2016
The Medicines and Healthcare products Regulatory Agency (MHRA) investigated an issue involving a digital calculator used by some GPs to assess the pot...

Ground breaking WEB RADR project marks mid point
Friday, 29 April 2016
The MHRA-led WEB-RADR project held its General Assembly in London on April 21...
