Medicines and Healthcare products Regulatory Agency
786 articles
Showing 751-780

New members sought for the Advisory Board on the Registration of Homeopathic Products
Tuesday, 24 March 2015
MHRA is looking for 2 members to the Advisory Board on the Registration of Homeopathic Products (ABRHP)....
Finding MHRA content on GOV.UK
Monday, 23 March 2015
A short video to help you find MHRA information on GOV.UK....

Certificates of Free Sale for medical devices: update
Friday, 20 March 2015
From 1 April 2015, MHRA will be issuing Certificates of Free Sale for medical devices. ...
MHRA is 1 of 10 regulators to help with the development of treatments for dementia
Monday, 16 March 2015
Following a workshop with other international regulators, MHRA has published information on how we continue to support the development of drugs for de...

Certificate of Free Sale to be issued by the Medicines and Healthcare products Regulatory Agency
Friday, 13 March 2015
The Medicines and Healthcare products Regulatory Agency (MHRA) will start issuing Certificates of Free Sale (CFS) from Wednesday 1 April 2015. ...

MHRA grants first scientific opinion
Wednesday, 11 March 2015
Dr Ian Hudson, Chief Executive of Medicines and Healthcare Products Regulatory Agency, comments on the first scientific opinion granted in the UK....

Regulator launches learning module on steroids
Friday, 06 March 2015
Online learning module on reducing the side effects of steroid medicines to help clinicians to optimise the use of corticosteroids....

An innovative approach to developing malaria vaccine
Tuesday, 03 March 2015
MHRA helps researchers at the University of Oxford take a step closer to developing effective vaccine against Malaria....

Sad death of MHRA Non Executive Director Professor Barrington Furr
Monday, 02 March 2015
It is with deepest regret that we announce the death of Professor Barrington Furr, one of our non-executive directors, who passed away on 27 February ...

MHRA network outage now resolved
Friday, 27 February 2015
The following is a service update....

Inaccurate TV advertising just one of the complaints upheld by the medicines regulator last year
Tuesday, 24 February 2015
MHRA has published the 9th edition of the advertising report Delivering High Standards in Medicines Advertising. ...

New chair appointed for the newly formed Devices Expert Advisory Committee
Wednesday, 18 February 2015
Dr Peter Nightingale has been appointed as chairman of the Medicines and Healthcare products Regulatory Agency’s (MHRA) Devices Expert Advisory Commit...

Hormone replacement therapy (HRT) and the risk of ovarian cancer
Friday, 13 February 2015
MHRA statement in response to the study in The Lancet on the use of HRT....

MHRA statement in response to Royal United Services Institute (RUSI) report: ON TAP
Thursday, 12 February 2015
Press statement in response to RUSI's report on organised crime and the illicit trade in tobacco, alcohol and pharmaceuticals (ON TAP) in the UK. ...

Warning of impersonation of MHRA staff
Wednesday, 11 February 2015
We are aware of telephone calls made to a company recently from an individual who does not work for the agency claiming to be a member of MHRA staff. ...

Kentish man given suspended sentence for sale of unlicensed erectile dysfunction drugs
Tuesday, 10 February 2015
A man has been given a 4 month prison sentence at Southwark Crown Court, suspended for 2 years, for the sale and supply of unlicensed erectile dysfunc...

Regulator warns against GcMAF made in unlicensed facility in Cambridgeshire
Tuesday, 03 February 2015
The Medicine and Healthcare products Regulatory Agency (MHRA) are today warning people who may have purchased an unlicensed medicine called GcMAF labe...

MHRA helps Japanese pharmaceutical company to develop its first European site
Thursday, 29 January 2015
It is hoped that the single site will help to improve the lives of patients and their families by developing medicines as quickly as possible....

Nexium Control 20mg Tablets now available for general sale
Tuesday, 27 January 2015
Reclassification of Nexium Control 20mg Tablets announced by the Medicines and Healthcare products Regulatory Agency today (27 January 2015)....

GVK Biosciences: MHRA responds to European Medicines Agency’s recommendation to suspend medicines
Friday, 23 January 2015
MHRA's response to EMA's recommendations to suspend medicines....

HRA and MHRA welcome public consultation on application of transparency rules of EU Clinical Trial Regulation
Thursday, 22 January 2015
Health Research Authority (HRA) and Medicines and Healthcare products Regulatory Agency (MHRA) welcome public consultation....

Stronger advice on the use of valproate medicines in women
Wednesday, 21 January 2015
Healthcare professionals are today being urged by MHRA to give women better information on the risks associated with valproate medicines....

New vice chair appointed to Commission on Human Medicines
Monday, 19 January 2015
Dr Angela Thomas replaces Professor Ian Weller who retired from the position and from CHM on 31 December 2014....

Regulator asks those with diabetes to check insulin pumps
Friday, 16 January 2015
MHRA is asking people with a particular insulin pump to check the clock resets to the correct time and date when they change the battery or when the p...
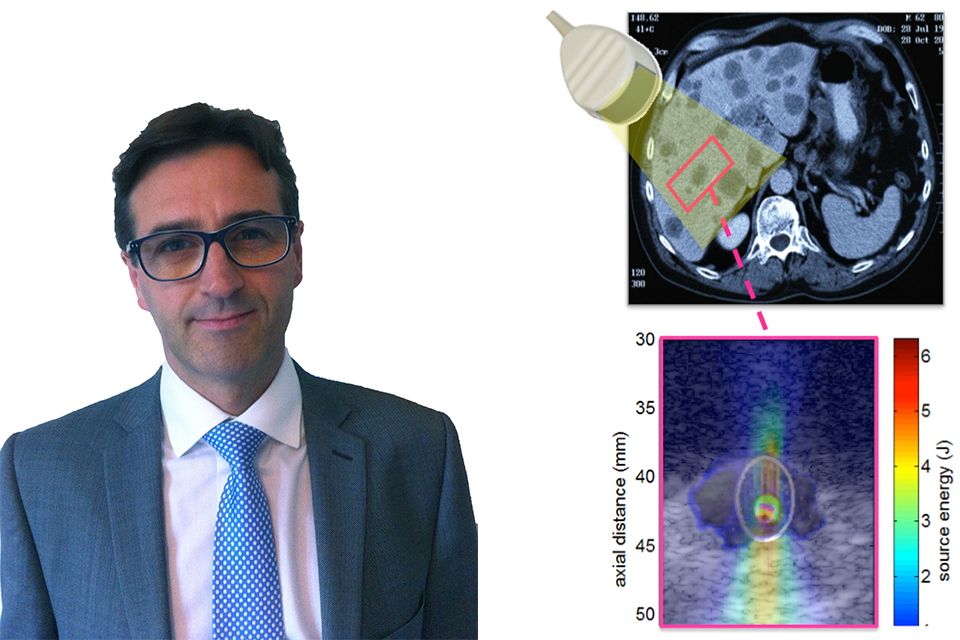
New case study illustrates how MHRA and OxSonics help cancer drugs to go the extra mile
Thursday, 15 January 2015
How MHRA and OxSonics help cancer drugs go the extra mile....

Diclofenac tablets now only available as a prescription medicine
Wednesday, 14 January 2015
People will no longer be able to purchase diclofenac tablets, used to treat pain and inflammation, from pharmacies without a prescription from their d...
Websites closed in battle against illegal trade in medicines
Thursday, 08 January 2015
MHRA took action to close down more than 1,600 websites illegally advertising and selling medicines last year....

New essential Orange and Green Guides 2015 – out now!
Tuesday, 06 January 2015
Latest UK pharmaceutical regulations, EU directives and guidance for manufacturers and distributors of human medicines available to buy now....

Dr Janet Valentine appointed as new CPRD Director
Monday, 05 January 2015
MHRA announces the appointment of new Clinical Practice Research Datalink (CPRD) director Dr Janet Valentine with effect from today. ...

Faulty insulin pens recall
Wednesday, 17 December 2014
People with diabetes who use insulin pens manufactured by Owen Mumford are today being warned by the Medicines and Healthcare products Regulatory Agen...
