Medicines and Healthcare products Regulatory Agency
782 articles
Showing 61-90

Vorasidenib approved to treat patients 12 years and older with grade 2 astrocytoma or oligodendroglioma with a susceptible IDH1 or IDH2 mutation
Tuesday, 16 September 2025
The Medicines and Healthcare products Regulatory Agency (MHRA) has today, 16th September 2025, approved the medicine vorasidenib (Voranigo). ...

Increased risks identified with Profemur cobalt chrome modular neck hip replacements
Wednesday, 03 September 2025
Patients implanted with affected devices should expect to be contacted directly for a follow-up assessment, if required....

MHRA approves UK’s first new type of antibiotic for urinary tract infections in nearly 30 years
Wednesday, 27 August 2025
As with any medicine, the MHRA will keep the safety of gepotidacin under close review....

MHRA approves zuranolone to treat postnatal depression in adults following childbirth
Wednesday, 27 August 2025
As with any medicine, the MHRA will keep the safety of zuranolone under close review....

MHRA launches Route B notification pilot as part of clinical trials regulations rollout
Wednesday, 27 August 2025
The pilot will help sponsors prepare for a new substantial modifications process under upcoming regulations, with responses delivered within 14 days....
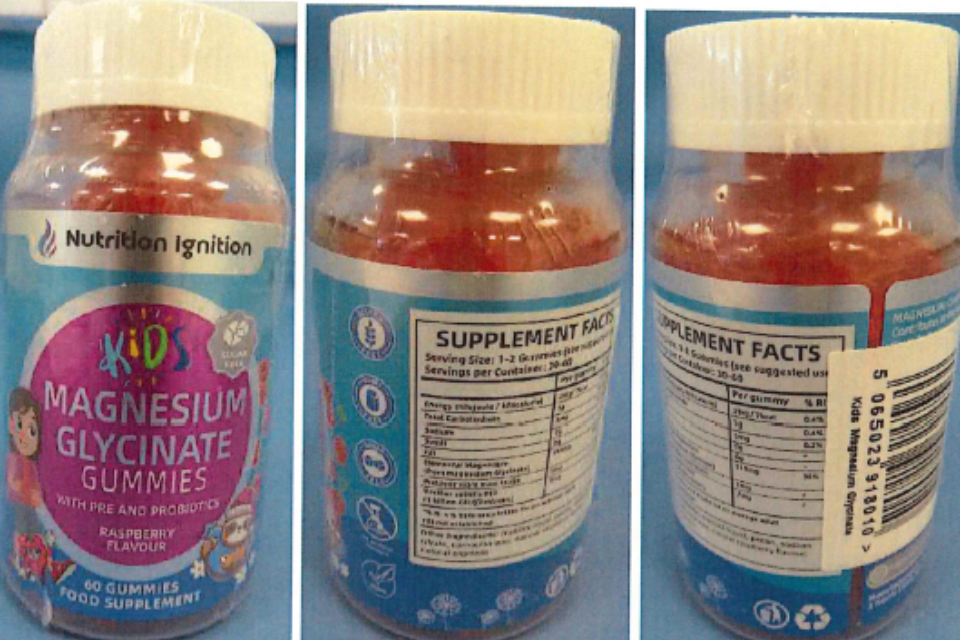
Parents and caregivers advised to stop all use of specific brand of kids’ magnesium gummies due to the presence of an undeclared prescription only medicine
Monday, 18 August 2025
Parents and caregivers who have purchased Nutrition Ignition Kids Magnesium Glycinate Gummies should stop giving them to children and safely dispose o...

MHRA approves teplizumab to delay progression of type 1 diabetes
Thursday, 14 August 2025
As with any medicine, the MHRA will keep the safety and effectiveness of teplizumab under close review. ...

MHRA designated as WHO Listed Authority: a milestone for UK life sciences and global health
Friday, 08 August 2025
WHO recognition affirms MHRA’s commitment to regulatory excellence, innovation and global collaboration...
New review highlights untapped potential of the vaginal microbiome in women’s health
Thursday, 07 August 2025
The microscopic bacteria living in women’s bodies could be a powerful tool for personalised, non-invasive treatment and earlier diagnosis....
Patients will receive medicines 3 6 months faster under 10 Year Health Plan, as regulators set out plans
Wednesday, 06 August 2025
Under a joint information sharing agreement, pharmaceutical companies will be invited to register early with the MHRA and NICE to allow parallel decis...

MHRA MedRegs blog – Summer 2025
Wednesday, 06 August 2025
Updates on regulatory reform and plans to support innovation....

MHRA begins recruitment for new digital roles at Leeds hub to drive innovation and smarter regulation
Monday, 04 August 2025
New jobs to strengthen innovation, safety and smarter regulation across the UK health and life sciences sector. ...

MHRA outlines intent to speed up patient access to innovative medical devices
Wednesday, 30 July 2025
Statement of Policy Intent sets out initial thinking on a new Early Access service to help patients benefit sooner from innovative medical devices tha...

Mirvetuximab soravtansine approved to treat adult patients who have ovarian, fallopian tube or primary peritoneal cancer
Monday, 28 July 2025
The approval is supported by a study involving 453 adults with advanced platinum-resistant cancers of the ovary, fallopian tubes and the peritoneum th...

Tofersen approved by the MHRA to treat rare inherited form of motor neurone disease
Monday, 28 July 2025
New genetic therapy approved for SOD1-ALS brings targeted treatment option to patients in the UK...

Yellow Card centre launched in Northern Ireland to strengthen patient safety
Thursday, 24 July 2025
A new regional centre to promote Yellow Card reporting has been launched in Belfast today....

Summer ready: MHRA issues updated guidance on medicines and medical devices during holiday season
Wednesday, 23 July 2025
As the UK enters the heart of summer – with temperatures rising and families holidaying –?the Medicines and Healthcare products Regulatory Agency (MHR...

Cutting edge personalised treatments, made while you wait, will deliver specialised care to patients more quickly
Tuesday, 22 July 2025
New regulations effective today will make it faster and easier for cutting-edge cancer treatments and personalised gene therapies to be made right whe...

MHRA announces proposals to improve access to world’s best medical devices for patients and to boost economic growth in Britain’s med tech sector
Tuesday, 22 July 2025
The MHRA has now published the government’s response to its public consultation on future routes to market for medical devices - designed to modernise...

The MHRA and the global flu vaccine: How the UK is helping shape the world’s flu vaccine
Tuesday, 22 July 2025
Ensuring the seasonal flu vaccine is ready, safe and effective involves months of international planning, testing and collaboration...

MHRA’s 2024–25 Annual Report and Accounts and Impact Report show progress on safety, innovation, and regulatory excellence
Monday, 21 July 2025
The Medicines and Healthcare products Regulatory Agency (MHRA) has published its 2024–25 Annual Report and Accounts, and accompanying Impact Report....

MHRA approves adrenaline nasal spray the first needle free emergency treatment for anaphylaxis in the UK
Friday, 18 July 2025
The Medicines and Healthcare products Regulatory Agency (MHRA) has today, 18 July 2025, approved adrenaline (epinephrine) nasal spray (EURneffy) to be...

Five non executive directors reappointed to the Medicines and Healthcare products Regulatory Agency Board
Thursday, 17 July 2025
Two board members have been reappointed for two years, while three others have had their term extended by a year....

MHRA CEO Lawrence Tallon welcomes Life Sciences Sector Plan
Wednesday, 16 July 2025
The Life Sciences Sector plan was released today (16 July 2025)...

MHRA approves sebetralstat (Ekterly) to treat hereditary angioedema (HAE) attacks in patients aged 12 and over
Tuesday, 15 July 2025
The Medicines and Healthcare products Regulatory Agency (MHRA) has today, 15 July 2025, approved sebetralstat (Ekterly) for the treatment of hereditar...

Government to align with European specifications on high risk in vitro diagnostic devices to reduce regulatory burden
Thursday, 10 July 2025
The specifications will establish standards for high-risk diagnostic tests while creating consistency with European regulations...

Don’t let the heatwave affect your medicines: Three important tips from the MHRA
Wednesday, 09 July 2025
Essential advice on protecting your medicines during extreme heat and staying safe this summer....

MHRA approves elinzanetant to treat moderate to severe vasomotor symptoms (hot flushes) caused by menopause ?
Monday, 07 July 2025
The Medicines and Healthcare products Regulatory Agency (MHRA) has today, 8 July, become the first regulator in the world to approve elinzanetant (Lyn...

MHRA approves nogapendekin alfa inbakicept to treat adult patients with non muscle invasive bladder cancer??
Friday, 04 July 2025
The Medicines and Healthcare products Regulatory Agency (MHRA) has today, 4 July 2025, approved nogapendekin alfa inbakicept (Anktiva) for adults with...

AI Airlock, CERSIs and a new global AI network for health regulators
Thursday, 26 June 2025
Med Tech Regs blog, June 2025: A focus on Software and AI....
